A group of anonymous persons filed a Statement of Concern with the FDA regarding the integrity of research papers about Simufilam, an Alzheimer’s Disease treatment drug candidate developed by Cassava Sciences (Nasdaq: $SAVA). The petition raises concerns about Western blots and methodology. I took a look at the problematic photos included in the report, and agree with most of those concerns. I also found some additional problems.
Disclosures
- I do not own any Cassava Sciences shorts or stock, nor longs or shorts or stock from other pharmaceutical companies that might be working on competing drugs.
- I was not involved in the publication of the Labaton Sucharow report mentioned below, and only heard about this case on August 25, after it was discussed on Twitter here and here.
- I was not paid by any person or organization to investigate these allegations, to analyze these papers, or to write this post.
- I get donations through Patreon for my ongoing work on science integrity, but no one asked me to work on the analysis in this blog post as a condition for their donation.
- This post is no accusation of misconduct, just a summary of image problems, some of which could be resolved if the researchers can show the original, uncropped data.
- This post is not meant to be financial advice. I am a scientist specializing in photographic scientific figures, and I know almost nothing about the stock market.
Simufilam
Simufilam is a drug candidate for the treatment of Alzheimer’s disease (AD). Initially called PTI-125, it is a small molecule that binds to Filamin A, a scaffolding protein that is involved in cell shape and movement. By binding to Filamin A, it can prevent the molecular events that increase tau phosphorylation, which is associated with neurofibrillary lesions found in AD.
According to Cassava Sciences, Simufilam treatment in Phase 2 clinical trials improved cognition in Alzheimer’s patients. This would be great news because there is no current treatment for AD. Cassava therefore hopes to quickly initiate Phase 3 trials, and that the drug will be approved by the FDA.
Statement of Concern
But last week, on August 18, 2021, Jordan A Thomas from Labaton Sucharow LLP (LS), a law firm in New York, posted a Statement of Concern report, asking the FDA to halt Cassava’s clinical trials because of “grave concerns about the quality and integrity of the laboratory-based studies surrounding this drug candidate.” The petition was filed on behalf of as yet anonymous persons.
The pre-clinical PTI-125 experiments have been conducted in the lab of Dr. Hoau-Yan Wang, an Associate Professor at City University of New York (CUNY). The work has been funded by Pain Therapeutics, Inc (PTI), the old name of Cassava Sciences. Wang currently serves on Cassava’s Scientific Advisory Board.
The LS report raised three types of concerns regarding the Simufilam/PTI-125 papers.
- Concerns about the integrity of the clinical biomarker data
- Concerns about the integrity of Western blot photos
- Concerns about the analysis of frozen human brain tissue samples
Here, I will focus on the Western blot concerns as raised in the petition, both in the papers supporting Simufilam, as well as in some other papers from the lab of Wang et al.

Western blot problems in Simufilam papers
The Cassava Sciences’ Publications page lists three Simufilam research papers and one review paper. According to the LS report, two of the three research papers contain Western blots with potential problems. I agree with many of these concerns and posted them on PubPeer – and also found some additional problems.
The three Simufilam research papers featured on the Cassava Sciences website are:
- Hoau-Yan Wang et al., Reducing amyloid-related Alzheimer’s disease pathogenesis by a small molecule targeting filamin A, Journal of Neuroscience 18 July 2012, 32 (29) 9773-9784 , DOI: 10.1523/JNEUROSCI.0354-12.2012 [PubPeer] [Funded by Pain Therapeutics]
- Hoau-Yan Wang et al., PTI-125 binds and reverses an altered conformation of filamin A to reduce Alzheimer’s disease pathogenesis, Neurobiol Aging 2017 Jul;55:99-114, DOI: 10.1016/j.neurobiolaging.2017.03.016 [PubPeer] [Funded by Pain Therapeutics]
- H.-Y. Wang et al., PTI-125 Reduces Biomarkers of Alzheimer’s Disease in Patients, The Journal of Prevention of Alzheimer’s Disease (2020), DOI: 10.14283/jpad.2020.6 [PubPeer] [Funded by NIH]
According to the LS report, several Western blots in the 2012 J Neurosci paper – in particular those with control proteins – show oversaturated bands with little background detail, irregularly spaced bands, and bands that look remarkably similar. I agree with these concerns and posted them on PubPeer. The blots are compressed and of relative low resolution, so I hope the authors can respond by showing the high-resolution scans to take away any concerns.

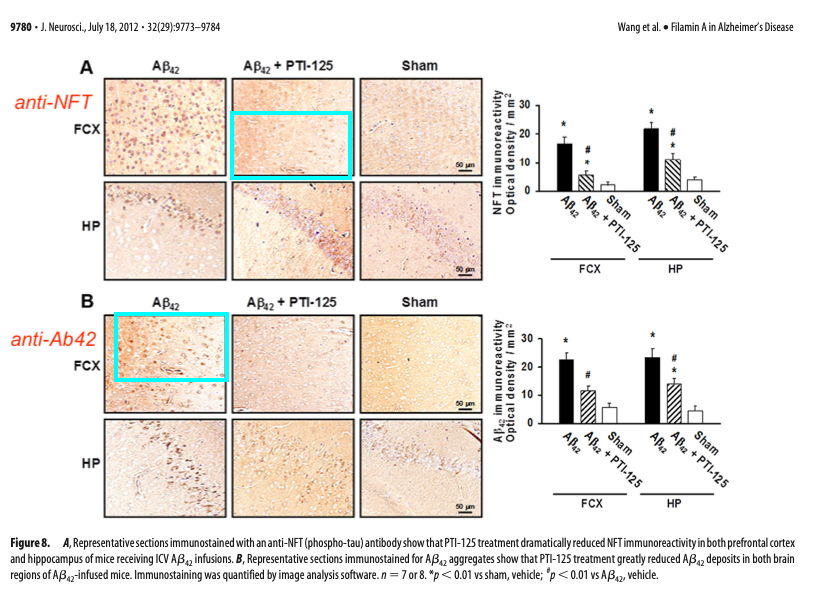
An additional concern in this paper, not mentioned in the report, is a potential duplication I found between two panels in Figure 8. Two panels displaying different immunostainings and treatment appear to share an area that looks similar. It’s indicated below with cyan boxes. These might be two consecutive tissue sections, perhaps stained for different proteins, although some features are remarkably similar in the right hand part of the image. However, the images are representing differently treated mice, so the samples should not look so similar.
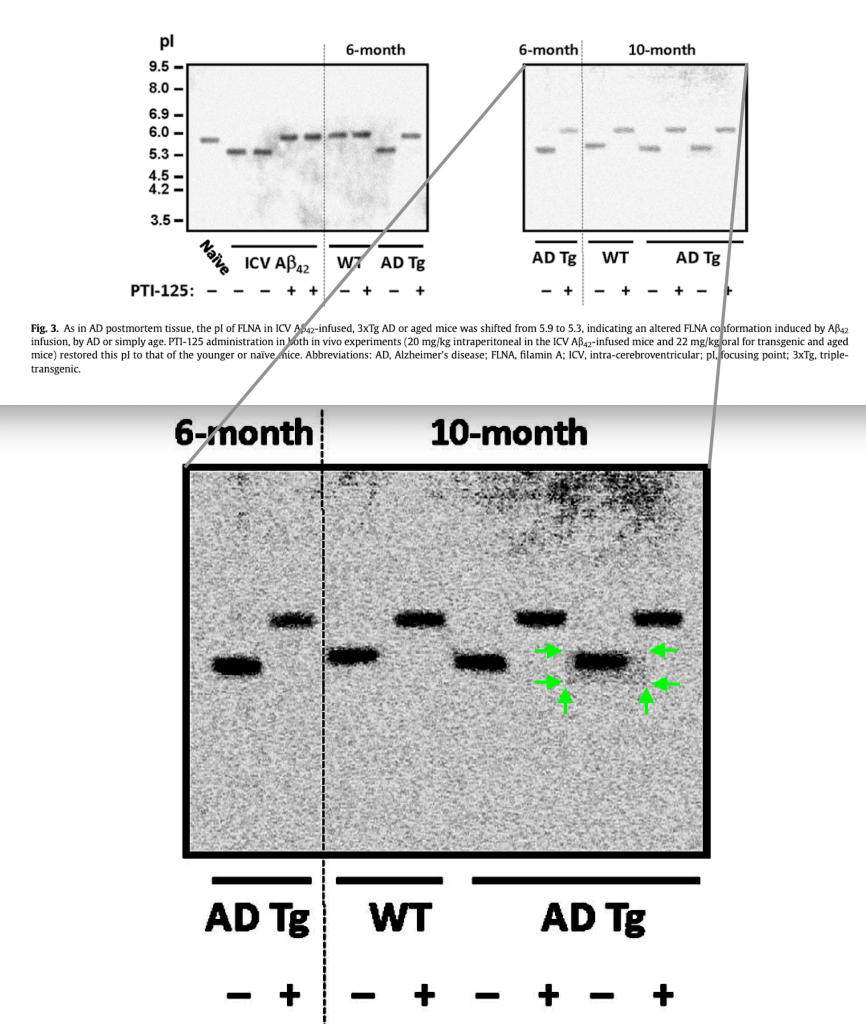
In the 2017 Neurobiol Aging paper, the LS report noticed some potential problems in Figure 12. In the top part, the NR1 blot contains only 12 bands, while 13 different conditions and lanes are shown in all the other blots. In addition, the NR1 blot appears to show a splice after lane 8, while the PLCgamma1 blot and most other blots show a splice after lane 10. This suggests that not all blots were derived from the same samples.
I agree with this concern and posted it on PubPeer. Of course, the authors could remove these concerns by showing the original, high resolution blots, and by explaining if and why the blots were spliced.

Statement of Concern”. Source: https://www.regulations.gov/docket/FDA-2021-P-0930/document
An additional finding by me – not mentioned in the LS report – is the presence of a dark rectangle around a band in Figure 3B. This could mean that the band shown in that lane may have been derived from another blot, making it hard to know exposure or size of the band relative to the other bands in that photo.
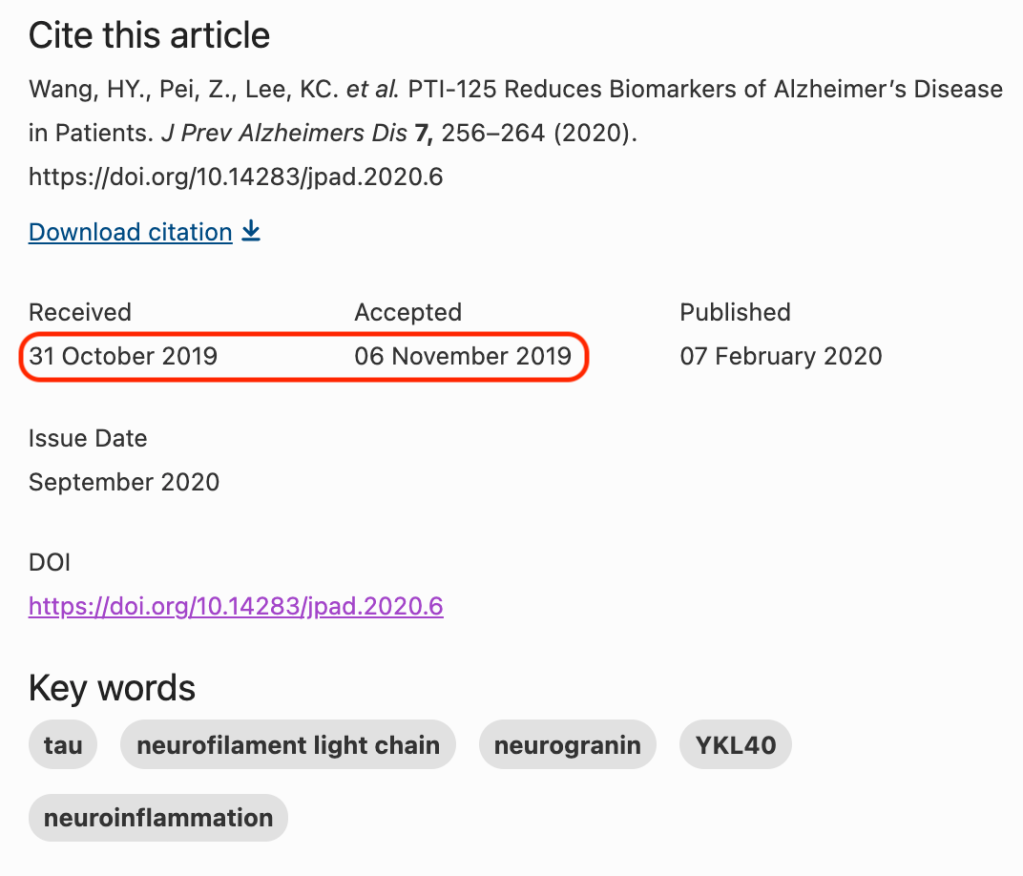
The third and most recent paper, JPAD 2020, was mentioned in the LS report because it was submitted to the journal on October 31, 2019, and accepted on November 6, 2019. Usually peer review takes anything from one to many months, so a peer review turned around in a week is remarkably fast.
The LS report did not mention any image problems, but Figure 3A of the paper appears to show a rectangular box around one of the pT231Tau bands. It is shown below with pink arrows. Some other bands, shown with blue arrows, appear to be surrounded by a white halo and to float above the background. The latter concern could just be a compression artifact, although it is not seen in all bands, just in a couple. And the authors can take away any concerns by showing the original blots in high resolution.

Other papers with image concerns
At least five other articles from the Wang lab at CUNY appear to show image concerns as well. These papers might not be directly related to Simufilam research, but they are still indirectly connected to Cassava Sciences or its drug candidates. Some articles are also about Alzheimer’s Disease or filamin A binding; some were funded by Pain Therapeutics, the Cassava Sciences precursor; and some are authored by Lindsay H Burns, the current Senior VP Neuroscience and lead scientist of Cassava Sciences. And of course, as mentioned above, Dr. Wang is one of Cassava’s Scientific Advisors. Together, the problems in these additional articles raise concerns about Western blots and perhaps also other data from this lab, spanning a period of 15 years.
The five additional papers are:
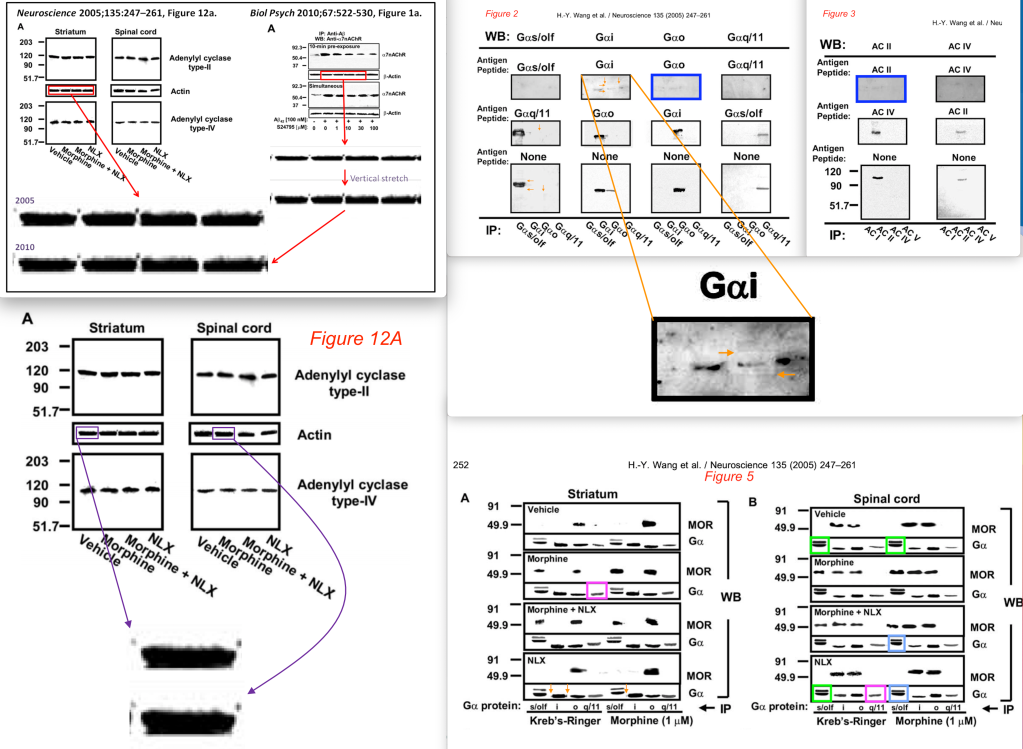
- H.-Y. Wang et al., Ultra-low-dose naloxone suppresses opioid tolerance, dependence and associated changes in Mu opioid receptor–G protein coupling and Gβγ signaling, Neuroscience (2005) 135: 247-261, DOI: 10.1016/j.neuroscience.2005.06.003 [PubPeer] [Funded by Pain Therapeutics, Inc]
- Jay J. Paquette et al., Cannabinoid-induced tolerance is associated with a CB1 receptor G protein coupling switch that is prevented by ultra-low dose rimonabant, Behavioural Pharmacology (2007), 18:767–776, DOI: 10.1097/FBP.0b013e3282f15890 [PubPeer] [Funded by NIH and Canadian grants]
- Hoau-Yan Wang et al., High-Affinity Naloxone Binding to Filamin A Prevents Mu Opioid Receptor–Gs Coupling Underlying Opioid Tolerance and Dependence, PLoS ONE 3(2): e1554 (2008), DOI: 10.1371/journal.pone.0001554 [PubPeer] [Funded by Pain Therapeutics, Inc]
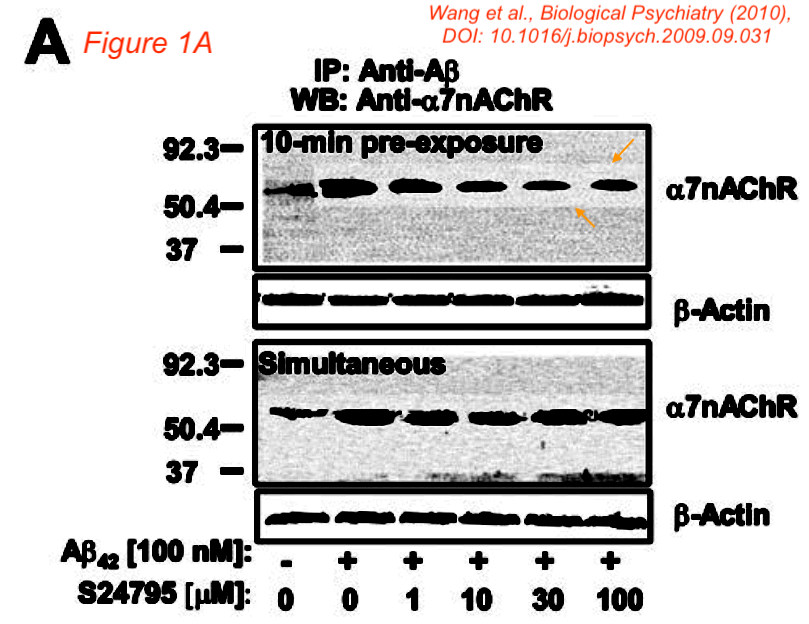
- Hoau-Yan Wang et al., S 24795 limits beta-amyloid-alpha7 nicotinic receptor interaction and reduces Alzheimer’s disease-like pathologies, Biological Psychiatry (2010), DOI: 10.1016/j.biopsych.2009.09.031 [PubPeer] [Funded by Servier]
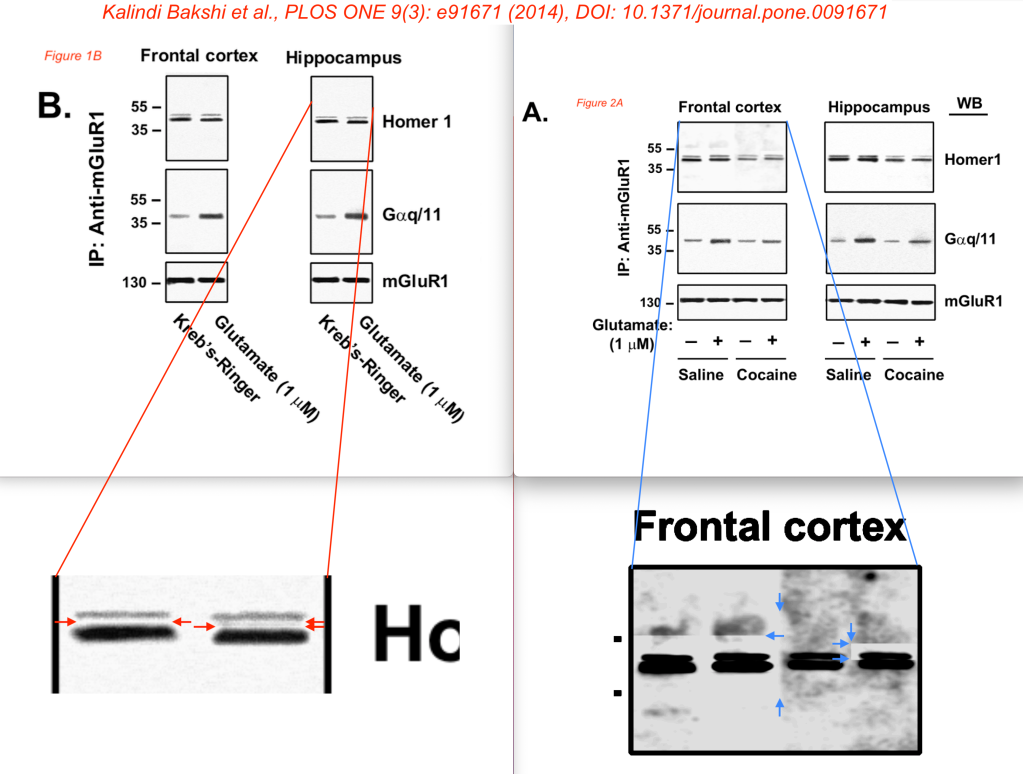
- Kalindi Bakshi et al., Prenatal cocaine exposure uncouples mGluR1 from Homer1 and Gq Proteins, PLOS ONE 9(3): e91671 (2014), DOI: 10.1371/journal.pone.0091671 [PubPeer] [Funded by NIH]
The Neuroscience (2005) paper has two blots that look similar; several bands that look similar; a rectangular box around a band; and a blot that appears to have been reused in the Biological Psychiatry (2010) paper, where it represented a different experiment. The collage below combines all findings, some of which were copied from the Report, plus some additional concerns that I identified.
The Behavioural Pharmacology (2007) paper was not included in the LS report, but Figure 4a appears to contain some duplicated bands. As always, making the original blots available might prove these bands are not the same.

Figure 7A of the PLOS ONE 2008 paper contains two blots that appear to look identical. In addition, some sharp background transitions suggestive of splicing may be visible.

An Actin blot in Figure 1a of the Biological Psychiatry (2010) paper looked, as mentioned above, remarkably similar to a blot panel from Neuroscience (2005), where it represented a different experiment. In addition, Figure 1A appears to show boxes around some lanes in the alpha7nAChR panel.
Finally, the PLOS ONE 2014 paper appears to show some problems in Figures 1B and 2A. These concerns were not part of the LS report, but are my own findings. Here, some blot bands appear to be surrounded by sharp transitions, suggesting that not all bands shown together in the same photo were derived from the same blot.
A rebuttal by Cassava Sciences
After the LS report was filed with the FDA on August 18, 2021, and publicly posted on August 23, Cassava Sciences wrote a response on August 25. From the press release:
“As a science company, we champion facts that can be evaluated and verified,” said Remi Barbier, President & CEO. “This helps people make informed choices. It is important for stakeholders to separate fact from fiction, which is why we wish to address allegations head-on.”
The parts of the response dealing with the problematic Western blots are not very convincing.
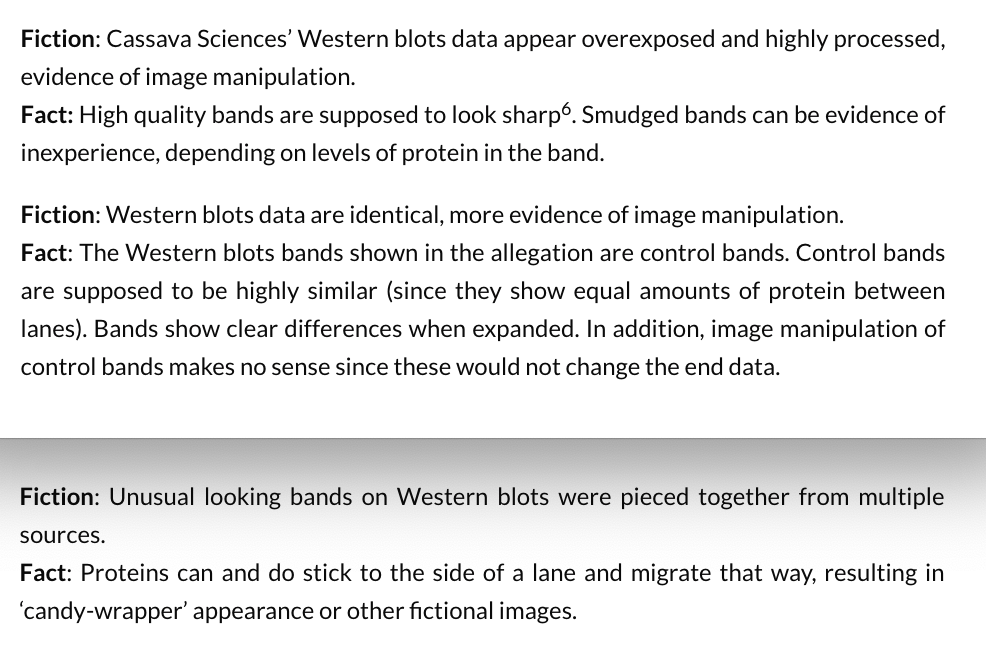
Rather than trying to dismiss the valid concerns with vague statements that “control bands are supposed to be highly similar” and calling the concerns “fiction“, I would like to see the authors present high-quality scans of the uncropped Western blot films. Showing original blots could take away some of the concerns, and might put the discussion to an end.

Hi, can you clarify – your statement says “A group of anonymous scientists filed a Statement of Concern to the FDA”.
I see a Citizens Petition from a law firm, is that what you are referring to? To quote the Bard himself – The first thing we do, let’s kill all the lawyers.
How can you state with such authority who is backing it?
Also, offhand, can you remember how many scientific advances were laughed at when they were first brought up for discussion, especially if they challenged the orthodoxy? I seem to remember when we burned people at the stake..
I have no idea whether Cassava’s drug works. And neither do you.
LikeLike
Who’s this “we”? I personally have never burned anyone at the stake. The papers by Wang and Burns sit well within scientific orthodoxy, which is how they were published so smoothly and with so few questions asked.
As a scholar of the Bard, you’ll recall that the line about ‘killing all the lawyers’ is spoken by a would-be tyrant, explaining the necessary steps for seizing absolute power.
LikeLike
She didn’t say whether the drug works or not. She’s saying that the data backing it up has serious problems. Your defensiveness regarding this drug says a lot.
LikeLike
Actually, she’s saying she thinks the data backing it up may have serious problems, but would like to see more to ascertain exactly.
I also said first “I have no idea” to highlight that I have no idea, and none of us do – that is the point of the drug trial.
After reading through everything several times this weekend, I think this lawyer is going to be reported by the FDA to several other three name federal agencies.
LikeLike
thx for sharing!
LikeLike
Statement of Concern
But last week, on August 18, 2021, Jordan A Thomas from Labaton Sucharow LLP (LS), a law firm in New York, posted a Statement of Concern report, asking the FDA to halt Cassava’s clinical trials because of “grave concerns about the quality and integrity of the laboratory-based studies surrounding this drug candidate.” The petition was filed on behalf of as yet anonymous persons.
WHY YOU ARE NOT MENTIONING THAT PETITION WAS FILED ON BEHALF OF SHORT SELLER (Short sellers make profit on declining stock price) AS REPORTED BY LAW FIRM ON LATER STATEMENT. PLEASE TRY TO BE HONEST IN YOUR ARTICLE. This petition lost credibility after that disclosure. I was very much interested in reading but lost interest after seeing the bias. Anyone can pull research of currently approved and effective drugs and find questionable points and can discuss it for weeks. I have list to share with you. I think we should let FDA do it part because they are the only one have full and detailed info in addition to Cassava sciences. It is not nice for scientists to be part of Short selling schemes. If i am wrong please accept my apology and correct me .
LikeLike
Dr. Bik – As a well-known medical image detective, a lot of us (who has no knowledge with the medical field) respect your opinion and rely on your expertise to make our decision. We trust that your analysis is the final truth because you wouldn’t publish it if you are not highly confident in it. We trust that you did your due diligence. I know you are fully aware that your analysis comes at a very critical time for SAVA and is currently aiding the short seller to inflict further pain on SAVA shareholders (you said a lot of $$ is at stake). I will work with SAVA to verify the data.
LikeLike
I’ll talk about this from an investor perspective and behavioral economics.
Most drug trials fail. Short sellers know this fact, and they bet on the statistical probability. They are mostly correct! Just a fact, that most drugs fail the trails. But when the short seller is wrong and the drug makes it, they can lose a lot of money.
Now, just because they are betting on the statistical fact doesn’t mean they are evil people that want this potentially amazing new drug to fail. But, if they have some industry knowledge and can smell BS they might take a bigger position and try to expose the BS because it will make them a lot of money.
The question here is are they ( law firm, short seller, other people that are loudly question validity) fabricating the BS or is there actually BS. The bad incentives here are hard to ignore.
There is a lot of money to be made either way, fabricating the good data, or fabricating the BS calling the data into question. Our author here doesn’t need to personally / directly make money of this articles implications, but her cousin or brother of friend from college can. Her patron can be generously donated to by the law firm or the short seller.
In any case, I’m sure the 3 letter agencies will have a field day with this one, and someone will end up in jail.
disclosure , I own a very small position in SAVA immaterial to my net worth. Mostly investing in it for hope that it will be a helpful drug and to stay on top of the science. If I was sure either way who is full of shit, I could make a lot of money. long or short either way, but it isn’t clear and the incentives for everyone to lie are very big.
LikeLike
Thank you for sharing.
LikeLike
You forgot to mention that this group of anonymous scientists ware backed by people with short positions. They admitted.
LikeLike
GASP! Thankfully, those defending $SAVA are doing so out of the goodness of their heart, and don’t have stock or options in this company.
LikeLike
Most of us do, sure, but – I know this might be hard for someone without a heart to believe, but get this – there are actually people out there that WANT the company/drug to succeed because they have a personal connection to someone with AD!!! I know, right? Absolutely insane! Imagine wanting a drug company to SUCCEED for personal reasons that aren’t financially motivated. Of course there are also some of us that found the company because of those reasons and then also realized it could be a lucrative financial opportunity. Or they want to help invest in the company in the hopes it might help them succeed. And yes, some of us are profit motivated. The short sellers on the other hand, are PURELY motivated by profit, and are potentially mucking with a drug that could benefit millions of people. Now I can only imagine what you are going to say – BUT THE DATA IS IN QUESTION!!! What about the harm of THAT ??? Well, I’ll quote a neurologist I know that looked into this – he said no AD patient that he has ever heard of was ever referred to them based off of biomarker data. That is just not how AD is diagnosed, and the numerous independent clinical trial results trump any potential issue with biomarker data being weird.
LikeLike
You’d like them to provide detailed scans from a 10-15 year old paper in a single night? A few hours after they saw the original complaint? While they’re initiating two Phase 3 studies? Just cause you don’t own stock, doesn’t mean you can’t be a friend of Biogen. Or Lilly. And just because a website calls itself Science Integrity don’t mean it’s got any.
LikeLike
Not sure where “in a single night” came from. The LS report was published on 18 August. Dr Bik’s blogpost is date-stamped 27 August.
LikeLike
The single night comment refers to SAVA’s response. About 12 hours after short law firm did it’s thing
LikeLike
An additional concern with 3A is that the bands you indicated don’t match the curvature of the blot. Every SDS-PAGE Blot I’ve done where there was that smile like curvature the bands at the edges would be slightly pulled down on the end closer to the center. You do see this on the second band in the image between the two you believe are concerning. I think that makes those figures that much more suspicious since the bands you’ve highlighted are flat and show no.such curvature. (I do know that PAGE is a different technique but the underlying physics is the same)
LikeLike
Have you looked at their patent which has a number of high resolution western blots.
Click to access US20140018341A1.pdf
I spent some time and the blots look mostly ok. Maybe the issues were indeed resolutions and trying to fit in stuff on the paper. What are your thoughts?
LikeLike
Also the company confirmed on friday that statistical data like Adas-Cog11 scores obtained at their trial sites are computed by independent agencies (did not name the agency). Their most recent 9 month data for 50 patients showed a cognition improvement of +3.1, which is unheard of. It could perhaps be a fluke but matches with the MoA of biomarker improvement for Alzheimers disease. The papers may have errors as you point out but I wonder if it was perhaps sloppiness or issues around performing complicated tests on brain tissue.
LikeLike
If the papers on which the clinical trials are based are falsified then the trial should not go forward. It does not matter what the motivations of the complainants are. Human lives are at stake in a clinical trial and that is the primary matter here.
All of the evidence is public. You can look at a lot of it yourself up above. The complainants could be monkeys banging on a typewriter, but still, those blots are extremely unusual, and they need an explanation.
LikeLike
The trial passed many significant end points and the data was verified by the FDA. The authors may yet clarify on any mistakes made and produce newer records. Papers can be corrected.
LikeLike
Human safety data the FDA used to okay P3 trials wasn’t based on western lots or the supposed altered images. It was based on human trials and further P2b trials.
Before recommending stopping trials based on western blots, you should look over the P2 trial data and understand the need for AD treatments.
LikeLike
I think any casual observer would agree the pictures show suspicious areas as you have described. However, I would be interested to know whether there are any circumstances where splicing results would be an acceptable practice or does it always indicate fraudulent intent when such splices are not identified by the authors. In the same vein, does it therefore invalidate the company’s stated patient impacts from taking simufilam. Thx.
LikeLike
Elisabeth M Bik is looking into western blot image manipulation by Dr. Wang. She prides herself in exposing fraudulent research. How could that be a bad thing, right? Then why is there a petition against Elisabeth’s work with thousands of signatures by fellow scientists? Because she operates as a prosecutor. She ignores reasons why the data has not been manipulated and highlights evidence that points towards manipulation. So the point is Elisabeth believes Dr. Wang is GUILTY until proven innocent. Well, you are the jury, and you should hear out the defendant before forming a conclusion. Specific to her opinions on Dr. Wang, I have some fundamental questions. When Elisabeth asks why the spacing between the bands are irregular, then I want to know exact measurements of the irregularity and what is considered normal (she hasn’t publicly stated what those are, and I doubt she knows either). She also failed to mention any other potential explanations, as an unbiased assessment should include.
The bottom line is that we have an AD epidemic that could be helped by this medication. It is about to enter highly regulated P3 trials. Over 12 months, there have been no significant safety concerns like death or hospitalization (you can’t hide that). Elisabeth’s actions are hindering the progress of that medication.
LikeLike
I’m a business lady, not a scientist. I have only one question to Mrs. Bik – who paid for her tremendous analysis – this job definitely took a lot of time and effords. Can’t be done for free. Any ideas?
LikeLike
Why can’t such a job be done for free, Olga?
LikeLike
Only idiots work for free, and she my friends is no idiot!
Show me the incentives and I’ll show you the results.
LikeLike
She said in the disclosure: She got donations through Patreon for her ongoing work on science integrity.
LikeLike
Dr. Bik,
I’m a writer for InvestorPlace, and I’m working on an article about Cassava. Our articles are often republished by Yahoo Finance and Nasdaq.com.
I apologize if I’m wrong, but it seems to me that you haven’t answered this question directly: Did any entity (including but not limited to a hedge fund and/or a law firm and/or a drug company) pay you to write this specific article and/or make one or more donations to your Patreon account with the expectation that you would research and write this article?
LikeLike
I did include a disclaimer in both blog posts about Cassava that answers that question. But I will answer it here again: No.
LikeLike
Then why is there a petition against Elisabeth’s work with thousands of signatures by fellow scientists?
Could you link to this petition, please? Are you sure you haven’t misunderstood the petition with thousands of scientists’ signatures supporting Dr Bik?
https://science.sciencemag.org/content/372/6546/1021.summary
LikeLiked by 1 person
There are petitions signed both supporting and questioning her. Pretty much like the debate over SAVA
LikeLike
Interesting analysis by a senior computer engineer:
https://discord.com/channels/807175385056936006/807180140840353802/881383769146286130
LikeLike
This link goes to a login page – can you provide a version for non-Discord-members, please?
LikeLike
It’s a very interesting analysis in PDF (21 pages) by a senior computer engineer and published 28th of August at Discord (full of pixel analysis; no simple copy/paste).
No idea how to upload that PDF here.
Summary
• On August 18, 2021, Jordan A Thomas from Labaton Sucharow LLP (LS)
posted a Statement of Concern report, asking the FDA to halt Cassava’s
clinical trials.
• On August 27, 2021, Elisabeth M Bik posted Cassava Sciences: Of stocks and
blots in Science Integrity Digest responding LS’s petition.
• I read EM Bik’s article and write down my comments.
• I don’t agree 14 of the 15 concerns raised by EM Bik. I’m not sure concern
#9.
• I don’t find any manipulation in Cassava Sciences’ publications.
LikeLike
Glad to hear that a computer engineer also is an expert in Western Blots and Alzheimer’s Drugs.
LikeLike
Hi! I have access to that report, for what it’s worth. If you give me a mail address or just write to the mail address below, I can mail it to you. Basically, the report just blows up those WB bands and concludes that they are not 100% the same, as a few – like 2 or 3 – pixels are always different…
LikeLike
Thank you. Someone else posted a link to a Google drive file and I am waiting for access to it.
LikeLike
The pdf version can be downloaded here: https://drive.google.com/file/d/1xdxExe9tPakKZawCYdsG5qFaftxbUS3H/view?usp=sharing
LikeLike
I have a Discord account but that link does not work for me. Can you provide more details on how to access it?
LikeLike
See https://drive.google.com/file/d/1xdxExe9tPakKZawCYdsG5qFaftxbUS3H/view?usp=sharing
LikeLike
This is a poor use of your time. You found a hit piece filed by a short seller scrutinizing images. You may have just as well posted an article in support of one of the most bullish cases of this drug made by a MEME maker. You will likely delete this article in the future when more facts are known. It adds nothing to what is happening.
LikeLike
Having worked and witnessed low or disgustingly absent integrity in the pharmaceutical industry with the likes of multiple Fortune 100 entities over 30+ years of my professional life, I pose a single clarifying question for Dr. Bik, said harbinger of integrity. Given the “push comes to shove” furiously displayed with this small molecule therapeutic poised to potentially provide tangible value to AD sufferers and knowing integrity ‘on any side’ can melt away or attempt to demonstrate its own “halo” effect:
What definitely should Cassava Sciences, Inc. do proactively beyond ‘in your view’ their initial public response, to put this matter firmly behind them?
I am certain, you would not want to play any tangible role that halts trial(s) of any agent for any potential benefit to our species knowing the limits of all our experience or stated expertise in applying scientific methodology anywhere along the continuum of discover to application.
LikeLike
They should post high resolution photos of all original blots that have raised concerns – if those are available. They should not brush away such concerns with words like “fiction” and “all bands look alike”. They should provide a good explanation about the “150%” data point that appeared to move from one group to another.
LikeLike
Enough about what “they should” do. Let’s talk about what you should do. You should not insinuate data fabrication without looking at the actual trial results. I can’t believe I have to state this. You should at least attempt to hear from a professional colleague before crucifying them on social media. I have to ask myself again, does this really have to be said?
LikeLike
being evaluated at a 3rd party lab as we speak
LikeLike
Question is: Can a computer engineer analyze the available low resolution computer images? (without being an Alzheimer expert)
Answer: Yes, a senior computer engineer investigated all the images in detail (even low resolution images that are compressed can be analyzed).
His conclusions are very different than yours:
Summary
• On August 18, 2021, Jordan A Thomas from Labaton Sucharow LLP (LS)
posted a Statement of Concern report, asking the FDA to halt Cassava’s
clinical trials.
• On August 27, 2021, Elisabeth M Bik posted Cassava Sciences: Of stocks and
blots in Science Integrity Digest responding LS’s petition.
• I read EM Bik’s article and write down my comments.
• I don’t agree 14 of the 15 concerns raised by EM Bik. I’m not sure concern
#9.
• I don’t find any manipulation in Cassava Sciences’ publications.
Pity I can’t upload the PDF (21 pages) here. It’s published 28th of August at Discord.
A question for now: Why are you hiding behind the availability of high resolution photos where analysis of the available images is simple and possible?
My conclusion for now is: You couldn’t analyze the current images correctly (and now are asking for high resolution images to hide incompetence in this) where the computer engineer simply did (experts have their expertise and you don’t have to be Alzheimer expert to analyze low resolution images correctly).
LikeLike
even low resolution images that are compressed can be analyzed
The trope of “Zoom and Enhance” is best left to CSI tv series.
LikeLike
Your logic of “high-resolution image” is awkward.
1) If the low-resolution images are identical, you may or may not find differences in high-resolution images.
2) If you’ve found differences in low-resolution images, the high-resolution images must be different.
LikeLike
Dr. Bik has great credibility and her concerns should be taken seriously. She offers a way out using the original data (which is pretty old in some cases and which may not be obtainable). What I find quite objectionable in the original request from the law firm, is that they ask that the current clinical trial of Simufilam be STOPPED.
This is appalling. The data in question is about laboratory results, not clinical response. Cassava’s data shows a degree of clinical improvement at 9 months. This is unheard of in Alzheimer’s disease, and something I never saw over decades as a clinical neurologist. Granted that Alzheimer patients (and all dementia patients) fluctuate from day to day), the response at 9 months is impressive and absolutely must be checked out by a placebo controlled trial of Simufilam (the design of which has just been cleared by the FDA and which is about to enter patients).
Actually, I think that Cassava’s data is even better than they realize — https://luysii.wordpress.com/2021/08/25/cassava-sciences-9-month-data-is-probably-better-than-they-realize/ based on my clinical experience with Parkinson’s disease.
If you want to see just how bad current Alzheimer therapy is, and how little evidence there is for the approval of Biogen’s drug have a look at this — https://science.sciencemag.org/content/sci/373/6555/624.full.pdf. Certainly it would be in Biogen’s interest to stop a trial of an orally available drug which appears to offer some benefit in an open label trial. The trial has been criticized for not being placebo controlled, but all preliminary drug trials are like this. For a concurrent placebo control with which to compare Cassava’s results I suggest the following. https://luysii.wordpress.com/2021/08/30/what-controls-should-cassava-sciences-use-for-their-open-label-trial/
LikeLiked by 1 person
I’m not a medical professional, but I think your comments are 100% correct. I was just writing a column for investors with very similar ideas.
LikeLike
Everyone can see for themselves the curiosities being discussed. There is not any serious question; the Western Blot images are not as expected and the dot plots do not match up fully with spaghetti plots. Cassava’s public replies were amazingly unprofessional and unhelpful; they did more harm than good. They confuse the term identical with similar. They argue that WB bands which are not sharp may be due to inexperience. Did a skilled individual put those presentations together? A skilled scientist? I believe that a skilled scientist could not have done them. A skilled graphics person with a Photoshop program? Possibly, but not a lab person. If you were a scientist really attempting to fool other scientists, the manipulation job would have been done at a much higher level, sort of like at the skill level of copying the Mona Lisa. The identified irregularities are way too obvious. One side is saying it is fraud, which makes no sense from the motive perspective. The other side has said there’s nothing wrong when there clearly is. No sense blaming people who noticed. The FDA will have to see the original Western blots. I suspect we will find out that the person tasked with putting together the posters the presentations and all the graphics was not skilled either in putting together scientific presentations or creating convincing deceptive ones. The person from Cassava responsible for the questionable responses was also not appropriate for the task. If it is found that there is not simply incompetence on these points but that root data was manipulated, with intent to deceive, that will be truly epic.
LikeLike
Stop typing and short the stock. It’s getting boring already.
LikeLike
People and families with Alzheimer’s need a cure.
I hope that the reason for doubting and criticizing Casava Science is not related to personal feelings or money.
LikeLike
People and families with Alzheimer’s need a cure.
I hope the reason for doubting and criticizing Casava Science is not related to personal feelings or money.
LikeLiked by 1 person
Elisabeth Bik said she first heard about this on August 25th. Her blogpost was then released the 27th. I get that your an expert but damn that’s a lot of research to review in that short amount of time. Just something that caught my attention
LikeLiked by 1 person
I have similar questions as Paul above, and I wonder if you could satisfy us with possible takes on the particular issue you have mentioned, the “150% data point” that you see moved from one group to another. Specifically, what about the results and/or claims of that paper/research might have been or conceived to be altered by such a move from one group to another of that 150% data point. Along the same lines, how much of a gain in putting forward the researcher’s thesis or interest would such a manipulation achieve, and would the work involved plus the risk of detection been little, medium, or a great leap, likely to give an already associate professor at a major university and a younger colleague with a rather stellar background some pause for concern. The bottom line to what I am trying to get at is whether or not it is relatively reasonable to think under the circumstances that a manipulation took place or would it just blow one’s mind that people of such stature would stoop so low (and, again, to gain how much in your view — i.e., to become famous and advance a remarkable drug for AD [even knowing that based on any serious foul play, the likelihood of real success in reducing cognitive loss would be pretty meager such that the ‘fame’ would be fleeting). I know such has taken place — the poor soul who produced wholly incorrect breast cancer data/studies, and to a much lesser, even questionable extent, the woman who worked in Baltimore’s lab and who was perhaps only a terrible record-keeper. Though I have considerable admiration for what you do and the seemingly immense talent you have for doing it, I cannot say I do not feel you owe those you throw a negative light upon the responsibility to do all you can to have clarity about not only what might have occurred (as you usually do), but what context and meaning exists related to possible conclusions, as I and it seems Paul seem to want you to do.
LikeLike
I do not understand what your point is (it is very wordy, but I cannot seem to find a question, and I am not sure what breast cancer or Baltimore have to do with this topic), but I have approved your comment.
LikeLike
He’s speculating out loud – what are the odds Yang and Burns plotted together on a multi year (decade) scam they thought they could take thru Phase 3 of a human drug trial and get away with it?
A request: Can you do a post where you summarize the current state of research/thinking around the gut microbiome, aging, the Western diet (or not the Western diet, ie MIND or whatever) and Alzheimer’s?
Serious question, btw, and something I would value your take on rather than 10-15 year old dots.
Thanks.
LikeLike
To be blunt, my question is really quite simple —How can you possibly believe there was intent to deceive without providing context about what such a deception would achieve for what outcome and why would mature people who have already reached high professional status risk all to try the deceits you imply? I mentioned the 150% because you picked it as if you thought it would be hard to explain so I asked that you explain its significance such that one could determine, Oh it is very important for them to make the point the change allows them to do and they probably thought they would not get caught; or, they were so caught up believing they would save the world (and bipolar) that they threw caution to the wind. What you do not do at all is show any real likelihood of deceit ! All the blots and all else , unless you can give or answer what I am asking, could ALL be meaningless errors or lapses in critical review spread over 15 years. Where is the beef!—that is the question. Make the case that something, anything there at all is such a great misleader that someone with stature would risk besmirching themselves by doing what you are implying. And, if you cannot do so, you should admit it. As far as I can see, you of all people know the poverty of precision in western blots and their review—by authors and hordes of editors/ reviewers, as well as other errors over 15 years not being that unusual. So, if you think it is more gravitas, tell us why —say, see here, if this not so , then nothing they say has anything to back it up so they had to lie because their careers were going nowhere and they were desperate. Make some real live sense about what you imply—that is what I ask. (Lastly to try to supply an example for you again, just tell us what would be different if they had not had any Western blots or what is so essential about the blots—that is, what if the research had taken place and the drug given 25-40 years ago and yielded the same result. Thanks for all your time. I know this might long and windy but e when in some ways two lives or at stake and in another millions of peoples lives, I like you, do not mind spending a lot of my time for free, and I would rather spend a lot then feel I had casually destroyed and disrupted these two researchers, their life’s work, and the possibility or timing of when millions might find some relief. (If you respond and if you wish, I will do you/pubpeer a favor, by posting entirely for free and your use, a relatively brief insight to as an aspect of their studies that is out there in plain sight but seems almost entirely ignored—more info on this if you want, including why I seem to be the only one to notice what I can make the case is the most important aspect of it all)
LikeLike
The short answer is that I do not know if there was an intention-to-mislead. All I see are blots that raise concerns, because the bands look very similar to each other, and/or are surrounded by boxes. There might be a perfectly reasonable technical explanation for this, and the authors can take away concerns by showing the original films or higher-resolution blots. So far they have not come forward with those blots.
With respect to your question why people want to deceive in science, my answer in general (without implying there is deceit in this particular case) is that it pays off too deceive. Positive results are easier to publish or easier to obtain grants/VC money with, than negative results. That is not only true in biotech or science, but it is true for any field. If results are not what one had hoped for, one might be tempted to make results look better, and that will be beneficial. Again, I am not saying that this is the case here. I am not in a position to investigate what happened. I only see blots that raise concerns and I am hoping the authors can show the originals and take away these concerns.
LikeLike
Can I get my last name removed from my comment, please?
LikeLike
Dear Dr. Bik, Cassava Sciences measured the oral bioavailability of simufilam at 100%. I find it hard to believe knowing that simufilam targets the same filamins and MOR/TLR4 receptors that are present in the gut and the brain. Naloxone, which was the prototype for simufilam, has only 3% oral bioavailability because of being trapped by MOR in the gut. Would you expect at least part of simufilam to be trapped in the gut? Thanks!
LikeLike
Dr Bik, we understand that you have been paid indirectly via your Patreon account in the form of a donation! it’s very clever but you take us for idiots to fire that you do this for free. In addition in 2 days you had time to do this !? You are not at all someone who is able to work for free, the proof you are complaining publicly that you cannot get paid for 2 hours of work in a French university! Very disappointed with your behavior, you only tried to do well paid FUD! Dr Wang doesn’t have to answer a FUD saleswoman!
LikeLike