Many image problems (duplications, manipulations) in scientific papers are found within Western blots. Several readers have asked me to explain what Western blots are, so here is a blog post written with the amazing help of Jon Cousins to provide some insights.
Western Blots are a standard laboratory technique used for studying proteins, one of the three basic building blocks of all living organisms. (The other two are DNA and RNA.)
The first of the three to be closely investigated was DNA, using a method devised in 1975 by British molecular biologist Edwin Southern, accordingly becoming known as “Southern Blotting.”
In the kind of joke that only scientists would ever dream up, the analysis tools later developed for RNA and proteins were correspondingly given the names “Northern Blotting” and “Western Blotting.”
(Purists may wish to note that, as there are only three building blocks, there was never any need for an “Eastern Blotting.”)
DNA – RNA – Protein
Three basic types of molecules in all living organisms (plants, animals, bacteria etc) are:
- DNA: a double stranded, corkscrew-shaped very long molecule that stores the genetic information. Together, the long DNA strands form the genome of an organism.
- RNA: a single stranded, corkscrew-shaped molecule. It has the same shape and information as DNA, but it is made of different molecules. It functions as a short-lived, temporary messenger molecule that can copy a piece of the information stored in DNA as a blue print to build new proteins.
- Proteins: globular, folded-up strands of amino acids that are either structural parts of a cell or enzymes that can carry out chemical reactions to make or break down other molecules such as polysaccharides or lipids.
In this great cartoon taken from a post written by Jaclyn Taroni on the Childhood Cancer Data Lab blog, DNA is like a cook book with lots of recipes, RNA is one single recipe, proteins are the dishes (and cupcakes!) that are prepared by following the recipes, while cells and tissues consists of many of these prepared dishes.

Studying proteins
Biologists who study proteins sometimes want to know how big proteins are, how much of a certain protein is found within a particular cell type or tissue, or how the amounts of certain proteins vary in cells as a response to a drug treatment or disease. To learn all that, they might use a molecular technique called a Western blot.
Step 1: Protein Gel Electrophoresis
Before a researcher can do Western blotting, the proteins need to be separated in a process called SDS-PAGE (which stands for Sodium Dodecyl Sulfate–PolyacrylAmide Gel Electrophoresis). In this technique, the protein samples are separated by length in a jello-like substance called a ‘gel’. There are several steps in this process:
- First, cells or tissues are homogenized and pretreated, so that each sample consists of a liquid mix of different proteins.
- The protein mixtures are mixed with a soap-like detergent solution called SDS to make them unfold and stretch, and to cover them with negative charges.
- The protein/detergent mixtures are loaded on top of a ‘gel’, a thin layer (~1 mm) of a jello-like substance called polyacrylamide poured between two glass plates. Multiple protein mixtures can be loaded next to each other in little holes called “slots” that are at the top of the gel (see illustration below).
- An electrical current is applied, with the positive pole at the bottom of the gel. As a result, the proteins, which are all negatively charged, want to move to the bottom of the gel. The samples slowly move from the top to the bottom of the gel, with each sample in a separate “lane”, like athletes running on a race track.
- The gel material however, is similar to a thick jungle, making it hard for the proteins to run to the positive pole at the bottom. Long proteins have a harder time running through this jungle than smaller proteins. Therefore, after a particular time of applying the electrical charge, the small proteins have moved much closer to the bottom than the larger and thus slower-moving proteins. The complex mixture of proteins thus gets separated according to length.
- When the smallest proteins have reached the bottom of the gel, the electrodes are removed, and the glass plates holding the gel are peeled from each other.
- The gel can now either be stained to show all proteins (e.g. with Coomassie Brilliant Blue or Ponceau dye), or the gel can be used to detect one or just a couple of proteins by Western blotting.

Western Blotting
During Western blotting, the separated proteins are transferred to a membrane and then incubated with a specific antibody to study one or a few proteins in the mix of many proteins in a homogenized sample. The membrane is much sturdier than the jello-like gel with the proteins, which can easily break or tear.
- First, the PAGE gel holding the proteins is put on top of a membrane, which looks like a small piece of paper. The membrane is made of nitrocellulose or PVDF, materials that proteins will easily bind to.
- The proteins in the gel are transferred to the membrane by “blotting”. There are several ways of doing this, either by putting a weight on top of the stacked gel/membrane and waiting until the proteins bind to the membrane, or by applying electrical current.
- The membrane, now called a “blot”, is incubated with an antibody against a particular protein of interest. Let’s say you want to study the amount of a protein called EGF. You would thus incubate the blot with an antibody against EGF, which is called “anti-EGF”.
- The antibody will bind at the position where EGF is located on the blot. If it is a small protein, the EGF will be located at the bottom of the blot.
- After incubating the blot with the antibody, the membrane is then rinsed, to wash away any unbound antibody.
- The membrane is then incubated with a secondary antibody that binds to the first, to amplify the signal. This secondary antibody has a radioactive or chemiluminescent groups that makes it visible on an X ray film or light-sensitive film or on a digital scanner.
- The film will now contain narrow horizontal stripes at the position where the initial protein of interest (e.g. EGF) had run. Usually, each lane will have one horizontal stripe. We call these stripes “bands”.

Gels, blots, lanes, and bands
In a protein gel, all the proteins present in a sample are separated according to length. In the image below, you see a gel with 6 “lanes” on the left. Lane 1 consists of an artificial, commercially available mixture of 10 known proteins of different sizes called the “Marker”. Lanes 2-6 consist of complex sample mixtures consisting of thousands of proteins of different sizes. The gel is treated with a cyan-colored dye that binds to all proteins.
On the right you see a duplicate of the gel on the left, one that has been transferred to a membrane and then incubated with a specific antibody, i.e. against a protein called CDK7. Although 1000s of different proteins are present in lanes 2-6, only the CDK7 protein bands are visible. (There are several “tricks” to make the marker band visible.) So even though the CDK7 protein was barely visible in the mixture of 1000s of other proteins, the Western blot technique allows us to study it with high precision and sensitivity.
The thickness / intensity of a Western blot band is a measure for the amount of that protein in a particular sample. The position in the gel (closer to the top or the bottom of the blot) is a measure for the size of the protein. Since the marker proteins – here in Lane 1 – are of known sizes, we can make an estimate of the size of the unknown protein.

Loading control proteins
A Western blot can be stripped to remove the antibodies, and then re-incubated with a different antibody to investigate a different protein of interest. This process can be repeated several times.
To make sure that all lanes have been loaded with the same amount of total protein, and in order to have a denominator for the amount of a protein of interest, one of those repeat Western blots might involve looking at a loading control protein. That is a protein that is expected to always be present in the same amount within a cell or tissue type, independent of the experiment.
Typical control proteins are beta-actin, GAPDH, AKT, or alpha-tubulin. These are “household” proteins that are essential for the functioning of eukaryotic cells. Often, the loading control incubation is shown at the bottom of a figure with multiple Western blot panels.
In the image below you can see that the amount of 4 proteins (A-D) varies between the different experiments (1-8), while the amount of Tubulin (bottom panel) remains pretty constant.
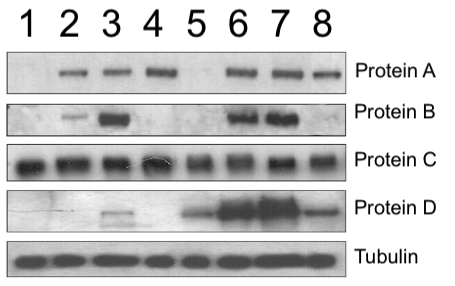
Every band should be unique
As you might already see by looking at the image in the previous paragraph, the shape and spacing of Western blot bands is quite variable. Bands can be shaped like pancakes, dumbbells, fishes, worms, clogs, bowties, W’s, M’s, smiling mouths, or whatever you might see in them. The shape depends on how the researcher loads the sample onto the gel, the amount of protein, and on the properties of the gel apparatus. Then you also might notice spots, stains, blotches, scratches, airbubbles, and other irregularities in the gel bands and background. All these variations cause bands to all look a bit different from each other. Like rocks or leaves, or faces, each band is somehow unique. Of course, this all depends on the resolution and exposure of the blot photo.
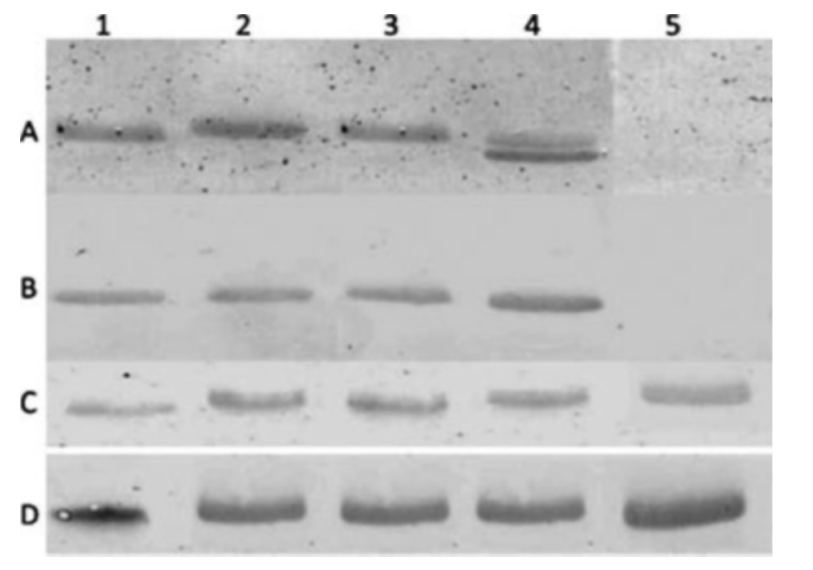
Because Western blot bands are supposed to be unique, one could visually inspect blot photos and detect bands that look more similar than expected – something that might hint at duplication or manipulation.
In the figure below, can you detect some bands in panels A and D that look more similar than expected?
Why is it called Western blotting?
The name “Western” blot is derived from an older technique developed in 1975 by Edwin Southern called “Southern blotting”. In Southern blotting, DNA is transferred to a membrane and detected using probes. Two years later, in 1977, a similar technique to detect RNA was called “Northern” blotting, sort of as a joke, but the name stuck. And when a similar technique was developed by three different labs around 1979 to transfer and detect specific proteins, “Western” blotting seemed to be an obvious name. And since there are only three basic molecules of life, there is no Eastern blotting!
Source Documents and Further reading
- Western Blot – Nature Scitable
- Western Blotting – MyBioSource.com
- Who really invented the Western Blot? – GenHunter Corporation
- SDS-PAGE – Sigma Aldrich
- What went wrong? A Western Blot Troubleshooting Guide – Precision Biosystems

Article is somewhat naive on image resampling in publication. Downsampling by definition will remove data from any image, and that means bands will lose data as the are translated from the raw data image into a smaller publication image form. See link to Adobe that will explain this to you in detail.
https://helpx.adobe.com/uk/photoshop/kb/advanced-cropping-resizing-resampling-photoshop.html
LikeLike