In my post on August 4 2020 I wrote about the mysterious Journal of Biological Regulators and Homeostatic Agents (JBRHA) that published the bizarre paper on 5G and Coronavirus (now withdrawn). Most papers in this journal are not accessible and the Editorial Board consists mostly of deceased people.
Browsing further back into the journal’s archive I found an interesting supplemental issue from 2016 that consists of 20 papers on psoriasis – all written by the same group of prolific authors.
The papers are not without problems. Lack of IRB approval, lack of patient consent to have their photos published, unclear patient recruitment and trial locations, inclusion of children in experimental drug testing, and to top it off, incorrect statements about conflict of interest. All papers heavily promote the same product line of herbal ointments and gels – and the founder of the company is one of the authors!
This is a huge conflict of interest.

The 2016 JBRHA Issue
The 2016 Volume 30, No. 2 (Supplement 3) issue of the Journal of Biological Regulators & Homeostatic Agents (JBRHA) contains 20 papers, all by the same group of authors (with minor variations), and all on the same topic, i.e. herbal products to treat skin diseases such as psoriasis or atopic dermatitis.
The 20 papers have PMIDs 27498651 through 27498670. A screenshot of the index page shows that most author names appear on all 20 papers.
No PDFs or full text options appear to be available through the journal’s website, but a PDF of the supplement, with all 20 full articles, was available on the FranklPharma website [archived].

Prolific writers
The authors of these 20 papers all appear to belong to the same group of 12 or so individuals, with some variations in the order. Faithful readers of science integrity blogs such as those of Sylvie Coyaud, Leonid Schneider, Smut Clyde, or mine will recognize some of these people.
Torello Lotti, an author on all 20 papers, and the corresponding author for 18, is a professor at the Università degli Studi Guglielmo Marconi in Rome, Italy. Although the Italian university lists him under the Department of Nuclear, Subnuclear, and Radiation Physics, other websites list him as Professor of Dermatology and Venereology. These fields are not immediately related, but maybe this professor is a Renaissance man.
In a recent blog post at ForBetterScience, Leonid Schneider reports that Lotti was arrested and charged with embezzlement for administering very expensive drugs in 2010. In that same post, Smut Clyde details the many journals of which Lotti is an Editor in Chief, and remark upon his association with omnivorous journals such as the JBRHA and the Open Access Macedonian Journal (OAMJMS) that gladly accept his papers by the dozen. Lotti currently has 956 papers in PubMed, most of which are case reports, reviews, or opinion pieces.
Uwe Wollina is chief dermatologist at the Städtischen Klinikum Dresden, in Germany. Despite his presumably busy practice, he had time to write a whopping 1,031 PubMed entries. Lots of his papers are case reports, featuring photos of women’s faces, often recognizable, and images of breasts and genital areas. Although full of fillers, these photos lack statements about patient approval. Dr. Wollina’s conveyor-belt-type production of papers was recently featured in Der Spiegel.
Massimo Fioranelli is a professor at the same faculty as Lotti. With only 110 papers indexed in PubMed, he is relatively modest in terms of productivity, but 101 of these papers were published in the last 5 years, so his star is rising quickly.
Michael Tirant is an Australia-based dermatologist who also has an appointment as a professor at the Guglielmo Marconi university in Rome. He opened several skin clinics in Australia, New Zealand, and Vietnam. He published 108 Pubmed-indexed papers.
Other authors found on all 20 papers in this issue are Jana Hercogová (176 papers in PubMed), professor at Charles University in Prague, Georgi Tchernev (384 papers), professor at the MVR in Sofia, Katlein França (116 papers), assistant professor at the University of Miami Miller School of Medicine, and Maria Grazia Roccia (90 papers), professor at the Guglielmo Marconi university in Rome.
This group of authors not only jointly published 20 papers in the 2016 JBRHA issue, they also often published together in other JBRHA issues, as well as in the OAMJMS, Dermatologic Therapy (of which Lotti is the Editor in Chief), and the Wien Med Wochenschr.
Sylvie Coyaud has written about their prolific collaborations here and here. After their 2016 collaboration, they were joined by Alireza Sepehri, resulting in perhaps the worst paper of 2020, “A Black Hole at the Center of Earth Plays the Role of the Biggest System of Telecommunication for Connecting DNAs, Dark DNAs and Molecules of Water on 4+N- Dimensional Manifold“, described in an earlier blog post, but now retracted.
Strong ties between authors and promoted drugs
All 20 papers in this JBRHA issue [list with PubPeer links] describe the efficacy and safety of a series of herbal products called Dr. Michaels® for the treatment of psoriasis and other dermatologic diseases. Just looking at some of the titles (Successful treatment…, Promising results…) it is no surprise that the papers all describe amazing improvements of clinical symptoms.
Several titles even prominently feature the names of these herbal skin care products. The Dr. Michaels products are sold by the company Frankl Pharma under the names Soratinex, Zitinex, and Eczitinex.
So how objective were the authors in determining the efficacy and safety of these ointments and shampoos? Were there any conflicts of interest?
Each of the 20 papers contains the following text: “Disclosure: All authors report no conflict of interest relevant to this article.”
But this statement appears incorrect. Several authors on the papers in this JBRHA issue have strong ties with the Soratinex / Dr. Michaels brand and with Frankl Pharma, the distributor of those products.
- Both Dr. Tirant and Dr. Lotti are listed on the Soratinex website under the Meet the Team page, suggesting these two authors work at / are paid by that company
- Dr. Tirant is listed on the website as the Soratinex founder and Principal Consultant
- Several Frankl Pharma websites (In Italy, UK, and Germany) state that the company represents Dr. Tirant and his products
- Dr. Tirant is listed as the creator or Erfinder of Soratinex on the Frankl Pharma websites
- Vice versa, the Soratinex products are featured on several sites of Michael Tirant, such as here and here.
- Uwe Wollina, Jana Hercogová, and Torelli Lotti, three other authors on all 20 papers, are featured on the Frankl Pharma websites as well
With at least two of the authors, Tirant and Lotti, so closely connected to the manufacturer and distributor companies of these products, there is a large conflict of interest.
On PubPeer I asked some critical questions. Why did the authors think there was no conflict of interest to declare? Could all authors please disclose how much money or other compensation (e.g. stock options, vacation trips, luxury goods) they received from Soratinex, Frankl Pharma, MT Dermaceuticals, or connected companies? Could the authors please also clarify how objective patient selection, patient inclusion, and assessment of disease severity were, with so many people connected to the tested products?
To be clear, there are situations in which employees or founders of a company can be an author of a scientific paper. But this should always be plainly disclosed. Hiding connections to such companies behind “no conflict of interest” statements is deceiving.

No control group
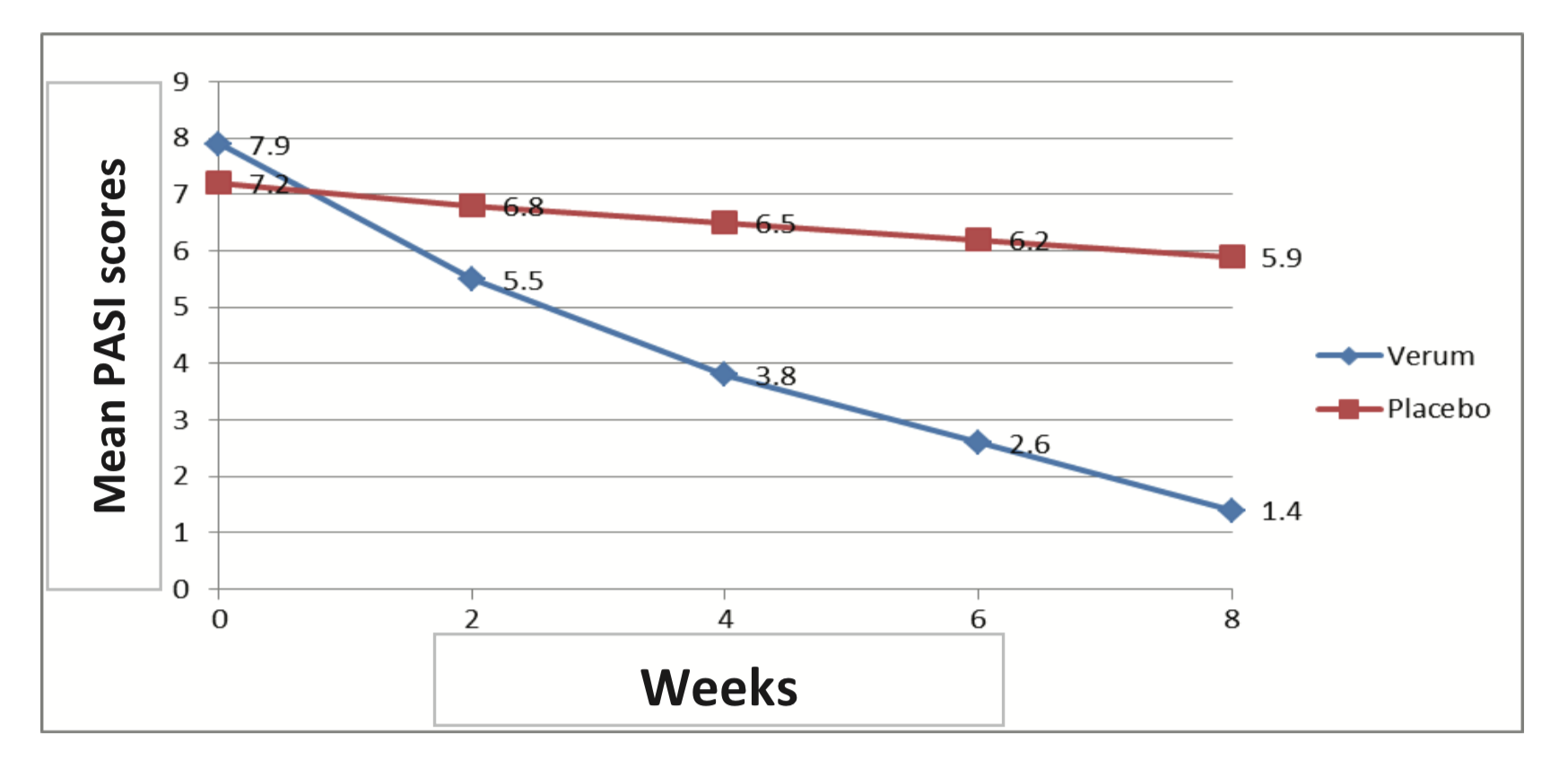
All 20 studies describe the successful treatment of a single patient or group of patients with Dr. Michaels products. However, most studies did not include a placebo/control group. In the one study that did include a placebo group,(França et al., European prospective, randomized placebo-controlled…, PubPeer), the placebo group also reported clinical improvement, albeit less dramatic than the verum group.
The graph was also lacking some error bars.
No IRB approval
Although several of these 20 studies report that the subjects signed a consent form, 19 of the papers include no language about approval by the Institutional Review Board of the hospital or clinic where the study was conducted. This seems a serious omission.
As with any experimental treatment, smearing ointments on damaged skin, such as found in psoriasis, is not without risks.
Some of the study subjects had to stop the treatment because of pruritus (itch) or folliculitis (inflammation of the hair follicles), which was reported by 4-23% of the subjects. Thus, the treatment is not free of side effects, and it is of concern that the authors conducted these studies without adhering to ethics board approval.
In addition, it was not clear if patients gave approval to have photos of their skin or face used in these scientific publications.
Several papers in this set describe the testing of the drugs on children, making the lack of IRB approval or parent/patient consent statement even more concerning.
Unclear study locations and author roles
In most of these studies, it was unclear at which hospital(s) the investigative work was conducted. This makes it hard to verify if patient consent and ethics approval (if any) has been performed correctly, or if assessment of disease severity was carried out objectively.
In one paper (Hercogová et al., PMID 27498653) a group of patients recruited at the Russian Pediatric Hospital Clinic and the Moscow City Hospital is described, despite the lack of any authors affiliated with Russian hospitals.
Another study (Gianfaldoni et al., PMID 27498667) included 62 patients from Romania, although none of the authors have an affiliation in that country.
In addition, it is unclear what each author’s role in these studies was. Some studies are case controls describing a single patient (PMID: 27498654, 27498656, and 27498665) so it is surprising that these papers were written by 12 or 13 authors, each from a different institution and/or a different country. How could clinicians from so many different locations all play a role in treating a single patient and writing a paper about that?
Skinterest
In conclusion, this set of papers appears to have been poorly reviewed, with unclear author roles, unclear patient recruitment, and unclear objectivity of the authors in assessing the treatment efficacy and safety.
Above all, several authors have not declared their strong associations with the companies manufacturing or selling these therapeutics. In particular, authors Michael Tirant and Torello Lotti are firmly connected to Frankl Pharma and/or Soratinex/Dr. Michaels and should have disclosed their conflicts of interest.
A list of the 20 papers with links to PubPeer comments can be found here (PDF).

Via Sylvie Coyaud’s blog again, I learned about “Textbook and Atlas of DERMATOLOGY” (M. Tirant, T. Lotti, D. Parsad). This seems to be a print-on-demand product and is available from one of Tirant’s web-stores for $180:
https://www.soratinex.com/product/textbook/
Also available for a much lower price through Amazon, where it is classified as “Hardcover Comic”. It is “An excellent aide in the diagnosis, treatment and management of skin diseases”, according to reviewer Prof. Torello Lotti. Purchasers are buying advertisments for various Tirant products:
“Preclinical and Clinical Studies on New Natural, Effective and Safe Treatments for Skin Diseases
Soratinex
Eczitinex
and other treatments”.
They are also buying an advertisement for “Artificial Hair: Automatic Biofibre® Hair Implant”, the subject of Chapter 44. It is no surprise that the tome has prime position in a webpage of academic advertisements for Biofibre hair replacement: https://www.biofibre.com/risultati/letteratura/
The page is dominated by papers from Lotti & Fioranelli et al, praising Biofibre. Perhaps they were cheapest.
LikeLike
Journal of Brouhaha:
https://pubmed.ncbi.nlm.nih.gov/26016977/ Polyamide hair implant (biofibre®): evaluation of efficacy and safety in a group of 133 patients
https://pubmed.ncbi.nlm.nih.gov/27373131/ Biofibre hair implant: what is new, what is true?
https://pubmed.ncbi.nlm.nih.gov/27373130/ Biofibre hair implant – impact on the quality of life
https://pubmed.ncbi.nlm.nih.gov/28702978/ The evolution of artificial hair implantation
Open Access Macedonian Journal of Medical Sciences:
https://pubmed.ncbi.nlm.nih.gov/29483977/ Automatic Artificial Hair Implant: Safety and Efficacy in Androgenetic Alopecia. A Prospective Study with a Highly Biocompatible Fiber
https://pubmed.ncbi.nlm.nih.gov/29484017/ Artificial Hair: By the Dawn to Automatic Biofibre? Hair Implant
LikeLike
Thank you for this article and the research you have done. I was considering trying soratinex after seeing it in an article and after researching online reviews (mainly success stories on the soratinex website, one of which was also posted on a psoriasis forum) and finding the scientific papers you have reviewed. I thought I could trust such papers but it seems as though this is not the case. After looking for further reviews on other websites I came across your article, and you have made me reconsider.
LikeLike